Ester And Co - Unpacking These Common Chemical Companions
Contents:
- Understanding Esters - What Are They, Really?
- What Are Esters - The Core Idea, and Co-existence?
- How Do Esters Form - A Look at Their Creation, and Co-products?
- What Kinds of Esters Are Common - And Their Co-occurrences?
- How Do We Name Esters - A System for Their Co-ordination?
- Where Do Esters Show Up in Daily Life - And Their Co-functions?
- Breaking Down Esters - What Happens When They Hydrolyze, and Co-reactions?
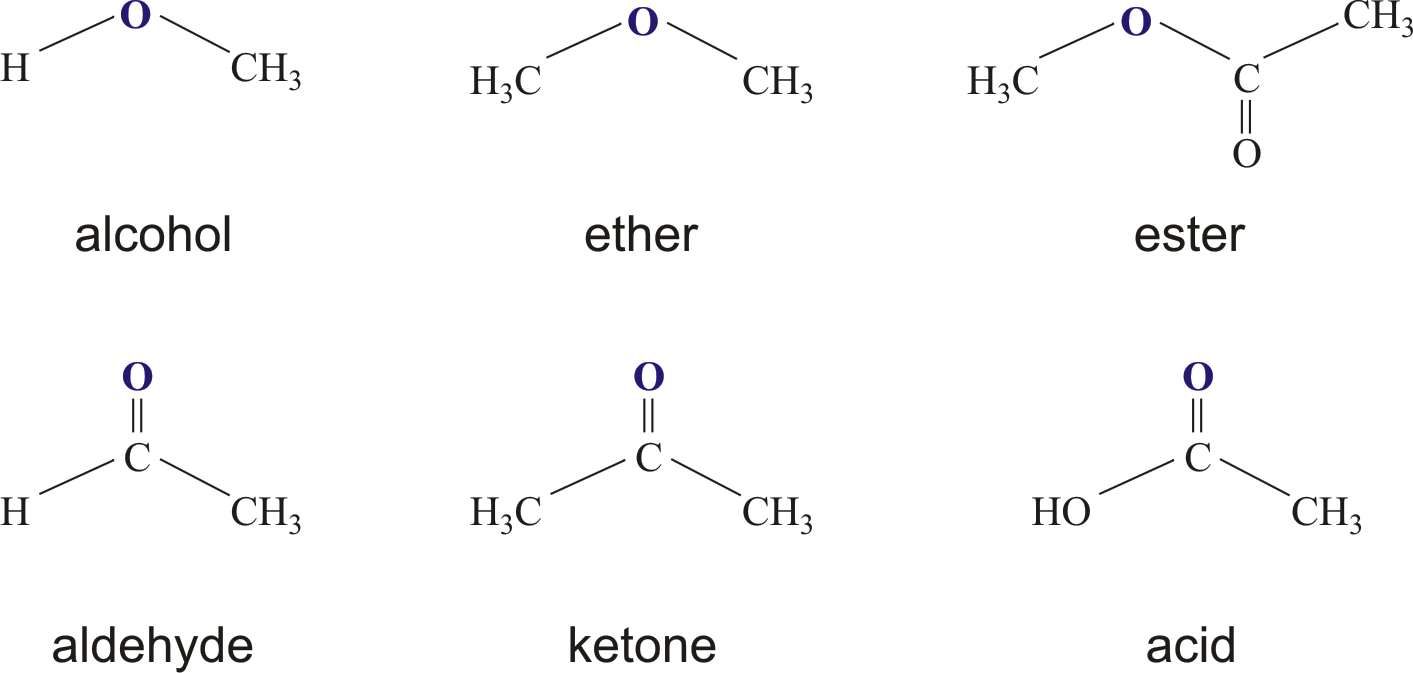
- The Parts of an Ester - Looking at Their Co-structure
Have you ever wondered about the hidden chemistry behind some of the most familiar things around us? You know, the smells of your favorite fruit, or the pleasant scent of a flower? There's a good chance a particular type of chemical compound, often called an ester, plays a big part in creating those experiences. These compounds are, in a way, like tiny architects of aroma and taste, present in many aspects of our daily lives, often without us even realizing it.
These interesting chemical creations are, in essence, built from other things, specifically from acids. It's almost like taking a building block from one structure and swapping it out for another. When we talk about an ester, we're looking at a compound where a specific part of an acid, a hydrogen atom from what's called a hydroxyl group, gets replaced by something else. This simple change, you see, makes all the difference in how the new compound behaves and what it can do.
- Fiona Gallagher Shameless
- Froot Vtuber Cheating
- Ralph Macchio Net Worth
- Lamar Jackson Injury History
- Iranian Sexism
So, these compounds are quite versatile, and they show up in various forms. While some are made from acids that come from living things, others can be put together from acids that are not organic at all. This means they have a wide range of possibilities for how they are structured and what they can be used for, which is, in some respects, pretty cool when you think about it.
What Are Esters - The Core Idea, and Co-existence?
An ester, in its simplest form, is a type of chemical compound that comes from an acid. It's like a relative of an acid, you might say. The way it comes into being involves a hydrogen atom, which is a tiny part of the acid's structure, getting swapped out. This hydrogen atom usually sits within a specific grouping of atoms called a hydroxyl group, and when it's replaced by another kind of atom or group of atoms, that's when you get an ester.
These compounds are, in a way, quite reactive when they meet water. When an ester mixes with water, it tends to break apart, and when it does, it produces two new things: an alcohol and an acid. This process is, basically, how you can reverse the creation of an ester, getting back some of the original pieces that went into making it. It's a bit like taking apart a toy to see its separate components, which is, you know, pretty interesting in the world of chemistry.
- Tails Comic Two Babies One Fox
- Baggiest Jeans In Atlanta
- 69069 Text
- Aishah Sofey New Leaked
- Daisys Destruction
Among the many kinds of esters, those that come from what are called carboxylic acids are, apparently, the most frequently encountered. These particular esters are very common, and you find them in a lot of places, both in nature and in things we make. So, when people talk about esters, they are often referring to this specific type, given their widespread presence and utility.
How Do Esters Form - A Look at Their Creation, and Co-products?
The creation of an ester involves a chemical process where two molecules come together and, at the same time, a water molecule is lost. This is often described as a condensation reaction. One of the molecules involved is an alcohol, and the other is an acid. When these two join up, they basically shed a bit of water, and what's left behind is the ester. It's a neat way that nature, or chemists, can combine different building blocks to create something new.
Specifically, a carboxylic ester, which is a very common type, is made this way. It's formed from a carboxylic acid and an alcohol. This particular combination is, you know, a very typical pathway for making these compounds. The process is, in fact, quite fundamental to how many organic compounds are put together, showing how molecules can interact and transform into different structures.
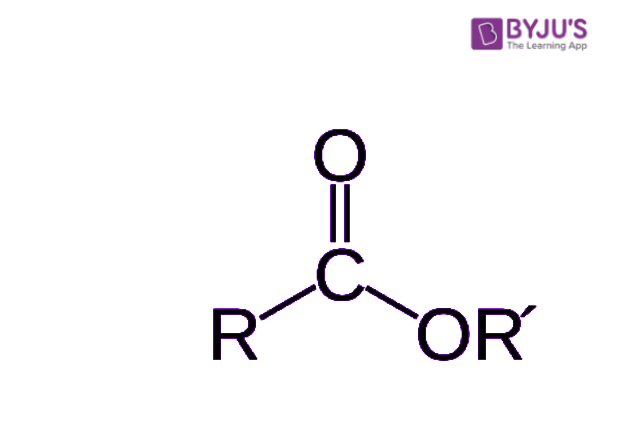
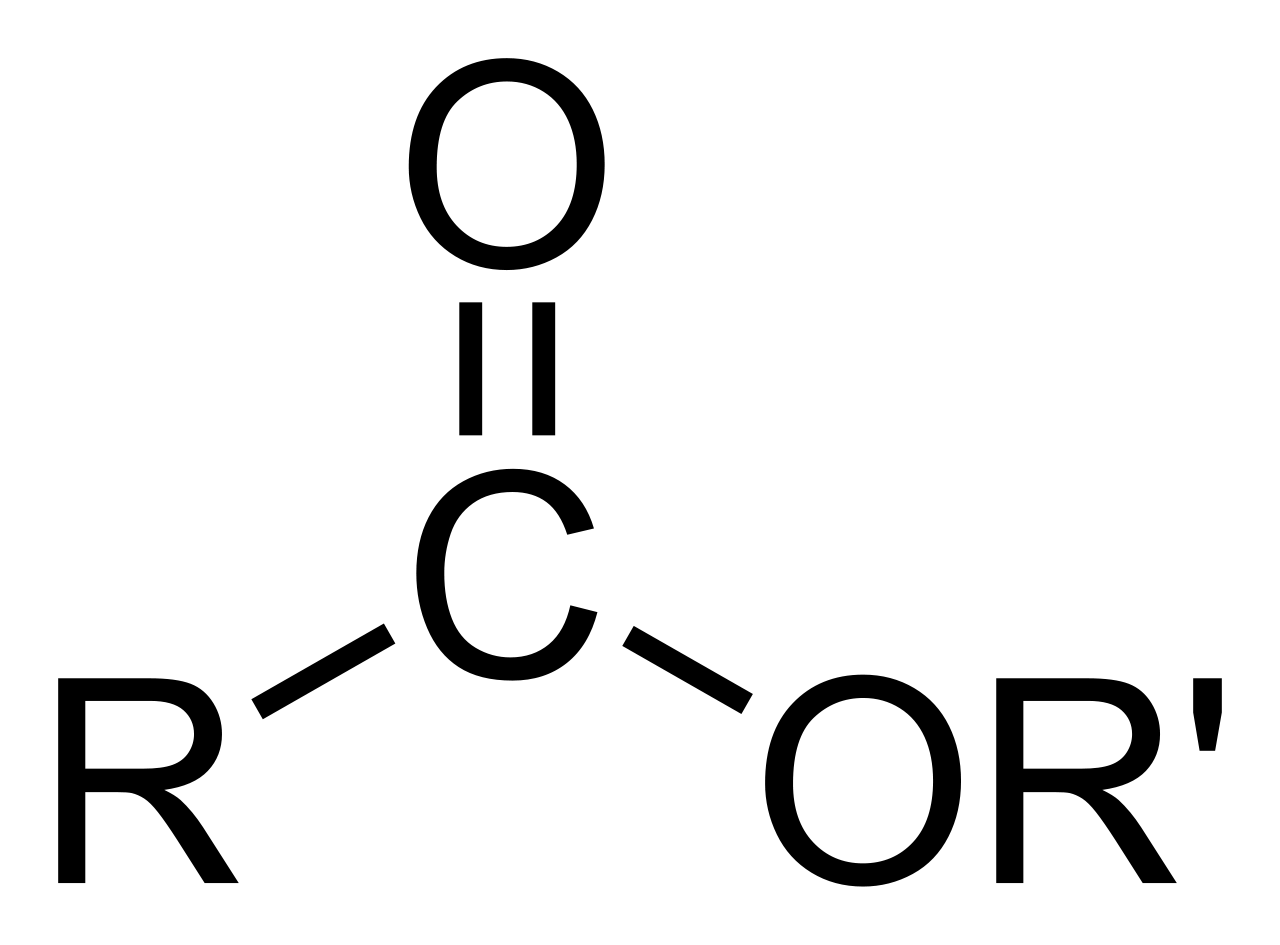
So, the general structure for an ester, once it's formed, has a specific arrangement of atoms. It's basically an organic compound where the hydrogen atom that would normally be part of the acid's carboxyl group is replaced. Instead of that hydrogen, you find a hydrocarbon group. This hydrocarbon group is, in a way, the defining feature that sets an ester apart from its parent acid, giving it its unique properties and allowing it to behave in specific ways.
What Kinds of Esters Are Common - And Their Co-occurrences?
As we mentioned, esters that come from carboxylic acids are, by far, the most common ones you'll encounter. These are the ones that typically come to mind when someone talks about esters in a general sense. They are found everywhere from natural sources to manufactured goods, showing just how often these particular compounds show up in our world. It's, in a way, a testament to their versatility and the ease with which they can be formed.
You find these types of esters, you know, derived from carboxylic acids quite frequently. They are, in fact, the workhorses of the ester family, appearing in a wide range of applications and natural phenomena. Their prevalence means that if you're looking at a compound that fits the general description of an ester, there's a really good chance it's one that started its life as a carboxylic acid before undergoing that specific transformation.
So, these common esters, with their specific make-up, contribute to many of the characteristics we observe in different materials. Their widespread presence means they are, in some respects, integral to many chemical processes and products that we interact with every single day. It's pretty fascinating, actually, how one type of compound can be so central to so many different things.
How Do We Name Esters - A System for Their Co-ordination?
When it comes to naming esters, there are a few ways to go about it, which helps chemists and others keep track of them. One common approach involves using what are called common names. These names are, you know, often simpler and might be used in everyday conversation or in certain industries, rather than strictly formal scientific settings. It's a bit like having nicknames for things, making them easier to talk about quickly.
Then there's a more formal way to name esters, following a system called IUPAC. This system is, basically, a set of rules that ensures everyone around the world names chemical compounds in the same consistent way. It's very precise and helps avoid confusion, especially when dealing with complex molecules. So, if you're looking for a really clear, unambiguous name for an ester, the IUPAC system is where you'd turn.
The names for esters, whether common or IUPAC, also include specific parts that tell you about the size of the carbon chains within the molecules. These parts are often called prefixes, and they give you a clue about how long the carbon backbone of the ester is. This information is, in fact, quite helpful for understanding the structure and, you know, predicting some of the properties of the ester, making the naming system a very useful tool.
Where Do Esters Show Up in Daily Life - And Their Co-functions?
Esters are, quite literally, all around us, and their presence is often tied to things we experience with our senses. One of the most significant places you find them is in the flavor and fragrance industries. Think about the sweet smell of a ripe banana, the distinct aroma of pineapple, or the pleasant scent of a rose. Often, it's an ester that gives these things their characteristic smell and taste. They are, in a way, the natural essence makers that we come across in our daily lives, making things like perfumes and food items smell and taste appealing.
They are used, for example, to create artificial flavors in candies, drinks, and other food products. So, that cherry flavor in your soda or the apple scent in a candle? There's a good chance an ester is responsible. This widespread use means that these compounds are, in some respects, key ingredients in many of the consumer goods we enjoy, contributing significantly to our sensory experiences.
Beyond flavors and fragrances, esters also have other roles, though the source text doesn't go into extensive detail. But it's worth noting that their chemical properties make them useful in various applications, showing their versatility. Their ability to contribute to so many different aspects of our lives is, you know, pretty remarkable when you consider it.
Breaking Down Esters - What Happens When They Hydrolyze, and Co-reactions?
Esters, while stable in many situations, can also be broken down through a process called hydrolysis. This happens when an ester reacts with water. It's a bit like the opposite of how they are formed. During hydrolysis, the ester molecule essentially splits apart, yielding two separate compounds: a carboxylic acid and an alcohol. This reaction can happen either with the help of an aqueous base or an aqueous acid, depending on the conditions. It's, in a way, a controlled method to reverse the esterification process.
When this hydrolysis reaction happens in a basic solution, it has a special name: saponification. This term comes from the Latin word "sapo," which means soap. The reason for this name is that this particular reaction, saponification, is the very process used to make soap. So, when you're using a bar of soap, you're actually interacting with the results of an ester hydrolysis in a basic environment. It's, you know, a pretty cool connection between chemistry and an everyday product.
This process of breaking down esters is, in fact, quite important in various chemical and biological systems. It allows for the recycling of components or the release of active substances that were previously part of an ester structure. The ability to break them down means that esters are not just static compounds; they can be transformed and repurposed, which is, actually, quite useful in many different contexts.
The Parts of an Ester - Looking at Their Co-structure
If you look closely at the structure of an ester, you'll notice a specific arrangement of atoms that gives it its identity. There's a central part, and then things branch off from there. One key feature is that a second oxygen atom within the ester molecule is connected to another carbon atom. This connection is, in a way, a defining characteristic of the ester functional group, making it distinct from other types of organic compounds.
This particular arrangement of atoms, with the carbon-oxygen-carbon linkage, helps to explain many of the ester's properties. It influences how the molecule interacts with other molecules, how it smells, and how it reacts. So, understanding this specific bonding pattern is, you know, pretty fundamental to grasping what an ester is all about and how it behaves in different situations.
The names we give to esters, as mentioned earlier, also reflect the lengths of the carbon chains that are part of these molecules. These names use prefixes that essentially tell you how many carbon atoms are in different parts of the ester structure. This naming convention is, basically, a way to describe the physical make-up of the molecule, allowing chemists to visualize its shape and size just from its name. It's, in some respects, like a shorthand for describing molecular architecture, which is, actually, very helpful.
So, to recap, esters are fascinating chemical compounds derived from acids, often playing a big role in the flavors and fragrances we enjoy. They form when acids and alcohols combine, losing water in the process, and the most common ones come from carboxylic acids. We name them using both common terms and a precise system that tells us about their carbon chains. They can also be broken down by water, a process called hydrolysis, with saponification being a well-known example that gives us soap. Their unique structure, particularly the way oxygen atoms connect to carbon, is key to their properties.
- Sowte Ifsa
- Desmond Doss The Unyielding Spirit Of A Conscientious Objector
- Emily Compagno Children
- Morten Harket The Voice Of Aha And His Enduring Legacy
- Is Riley Green A Republican Or Democrat
Esters @ Chemistry Dictionary & Glossary
Ester - Definition, Structure, Esterification along with Properties & Uses
Ester Definition, Examples And Facts | Chemistry Dictionary